the storage environment
1 Temperature and Relative Humidity | 2 Guidelines for Temperature and RH | 3 Pollutants | 4 Guidelines for Pollutant Levels | 5 Light | 6 Guidelines for Light Levels
|
|
All organic materials deteriorate, but research has shown that collections kept in a stable moderate climate will have a longer lifespan that those that are frequently subjected to hot and humid conditions. However, maintaining such a climate can be a challenge. Staff may be faced with older mechanical systems that are difficult and costly to control, systems whose target set points are dictated primarily by the requirements of human comfort, and/or facilities staff who are not sympathetic to the needs of collections.
In this section, you will explore best practices for controlling your institution's environment, following the recommendations of architects, HVAC engineers, collection managers, and preservation professionals. Standards for climate, pollutants, and light exposure in collection storage areas will be discussed, while considering sustainable approaches to maintaining an appropriate and efficient environment.
Later in this session you will learn how this optimal environment might be achieved within your building, as well as how you might improve existing conditions even if it is not possible to attain the ideal environment.
1 Temperature and Relative Humidity
Temperature can be defined as a measurement of how quickly molecules are moving within a material. As molecules move faster, they collide more frequently, making chemical reactions more likely. Molecules move more rapidly at higher temperatures, so heat accelerates the chemical reactions that cause deterioration. As a general rule of thumb, the rate of many chemical reactions is doubled with an increase of 18°F. Thus, a lower temperature means a slower rate of deterioration.
|
|
Relative humidity refers specifically to the amount of water vapor contained in the air at a given temperature, relative to the total amount of water vapor the air is capable of holding at that temperature.
The amount of water vapor in the air is important for two reasons: moisture provides fuel for the chemical reactions that cause deterioration (e.g., acid hydrolysis), and it causes physical damage such as swelling and shrinking. Organic materials such as paper naturally try to come to equilibrium with the surrounding air, so they absorb moisture as the relative humidity rises, and release moisture as it falls. Thus, a period of sustained higher relative humidity results in a quicker deterioration rate. Likewise, sustained periods of very low relative humidity can result in desiccation and cracking of some materials. Frequent fluctuations in relative humidity (and temperature) are even more damaging.
Temperature and RH are Interrelated
When managing climate within collections storage spaces, it is crucial to understand that air is capable of holding more water vapor at higher temperatures. Thus, given that the absolute amount of water vapor in the air remains the same (unless moisture is added or taken away through humidification or dehumidification), the relative humidity will go down if the temperature is raised, and it will go up if the temperature is lowered.
2 Guidelines for Temperature and RH
Over the past decade, climate change, soaring energy costs, and a conscious movement towards more sustainable, green approaches to energy consumption have dramatically changed the way that libraries, museums, and archives manage their environment. During the latter half of the twentieth century, air conditioning technology improved dramatically and targets for an "ideal" temperature and relative humidity evolved as a way of assuring an appropriate environment for collections in storage, exhibition, or on loan. The "50/70" rule --shorthand for conditions of 50% ±5% relative humidity and 70°F ±2° -- served for many years as the "ideal" setting for many materials in cultural heritage collections and was written into many building specifications, HVAC programs, and loan agreements.
The "Ideal" Preservation Environment: Magic Numbers
Few institutions could achieve the "50/70" rule, and those who did saw their energy costs double or triple in the last decade. Further, collection managers and preservation practitioners had to balance the fact that no single environmental setting was best for every collection; rather, the variety of formats (film vs. paintings; magnetic tapes vs. parchment documents) in libraries, museums, and archives have competing environmental requirements.
So while targeted temperature and relative humidity settings may sometimes be regarded as unrealistic "magic numbers," it is still worth considering the suggestions put forth by the 1995 National Information Standards Organization (NISO) technical report Environmental Guidelines for the Storage of Paper Records (NISO-TR01-1995). The temperature and relative humidity values suggested in the table below still serve as a strict "ideal," though more achievable and sustainable approaches have been developed in the years since.
|
|
SITUATION |
TEMPERATURE |
RELATIVE HUMIDITY |
|---|---|---|---|
| Combined stack and user areas |
70 °F maximum* | 30-50% RH** | |
| Stacks where people are excluded except for access and retrieval |
65 °F maximum* | 30-50% RH** | |
| Optimum preservation stacks |
35-65°F*** | 30-50% RH** | |
| Maximum daily fluctuation |
±2°F | ±3% RH | |
| Maximum monthly drift | 3°F |
3% |
* These values assume that 70°F is about the minimum comfort temperature for reading and 65°F the minimum for light physical activity. Each institution can make its own choice.
** A specific value of relative humidity within this range should be maintained ±3%, depending on the climatic conditions in the local geographic area, or facility limitations.
*** A specific temperature within this range should be maintained ±2°F. The specific temperature chosen depends on how much an organization is willing to invest in order to achieve a given life expectancy for its records.
Sustainable Preservation Environments
James M. Reilly, founder and director of the Image Permanence Institute, has defined the new optimal preservation environment as "one that achieves the best possible preservation of collections at the least possible consumption of energy, and is sustainable over time." This new thinking on programming collection environments takes into account global climate change, environmental responsibility, climbing energy costs, and emerging research revealing how collections react to fluctuations in temperature and relative humidity. While it was once believed that minimal (±3% RH) fluctuations in relative humidity accelerated deterioration of most collection formats, there is now substantial evidence (well documented in the AIC Environmental Guidelines Wiki) that materials are more physically resilient to relative humidity fluctuations than previously understood. Short-term changes, though not ideal, are typically insignificant because collections take time to equilibrate to a change in surrounding relative humidity.
For foundational advice on defining sustainable storage environments, read Reilly's "Specifying Storage Environments in Libraries and Archives" from the 2007 conference From Grey Areas to Green Areas. For the latest information on sustainable environments, check in with the Sustainable Preservation Practices for Managing Storage Environments website and review the emerging guidelines for museum loans developed by the AIC Environmental Guidelines Working Group.
3 Pollutants
Like temperature and humidity, the damaging effect of pollutants on collections can be difficult to see in the short-term, but very serious over the long term. Pollutants fall into two general categories, particulates and gases, and may originate outdoors or within the building.
Particulate Pollutants
|
Deposits of particulates around this ceiling air vent indicate that particulate filtration is inadequate. |
Particulates consist of organic and inorganic materials (e.g., hair, skin cells, soot, fibers, pollen, metals, salts, plaster, etc.) that may come from humans, smoke, cooking, construction materials, heating systems, and other sources. Particulates are categorized by size; fine particles have a diameter of 2.5 microns or less, while coarse particles have a diameter of 2.5-10 microns. Particulates are abrasive, they can attract insects, and many are acidic, reacting with moisture to worsen chemical deterioration. These pollutants may also provide a substrate for mold growth. The effect of particulate pollutants worsens in the presence of high relative humidity and temperature.
Gaseous Pollutants
Common gaseous pollutants in the outdoor environment include sulphur dioxide, which comes from combustion of fossil fuels, nitrogen oxides, mainly from traffic exhaust, and ozone, which produced by heavy electrical machinery, as well as when light reacts with nitrogen oxides. Indoor pollutants can also be a problem. Ozone is produced by photocopiers, laser printers, and electrostatic air cleaners. Acids, ammonia, formaldehyde, and peroxides are also produced by indoor materials such as cleaning products, particleboard, adhesives, and oil paints.
The amount of outdoor pollution that actually penetrates a building and affects collections is difficult to quantify, but certainly evidence has shown that collections in urban institutions are more acidic than those in institutions located in more rural settings. Gaseous pollutants can be unpredictable; even in fairly small concentrations they may interact with each other to cause damage.
4 Guidelines for Pollutant Levels
There are no formal standards for pollutant levels in collection storage areas, but generally accepted levels for particulate filtration are given in NISO's 1995 Environmental Guidelines for the Storage of Paper Records:
Combined stack and user areas |
60-80% filtration (e.g., remove 60-80% of particulates 1 micron diameter or larger) |
|
Stack areas, users excluded except for retrieval |
90-95% filtration |
|
Optimum preservation areas |
>95% filtration |
Generally accepted levels for the most common gaseous pollutants in collection storage areas, including sulphur dioxide, nitrous oxides, and ozone, fall in the following ranges:
Sulphur dioxide |
1-10 micrograms/cubic meter |
|
Nitrous oxides |
5-10 micrograms/cubic meter |
|
Ozone |
2-25 micrograms/cubic meter |
It is important to be aware that the upper limits for exposure of collections to pollutants are well below the acceptable levels for human exposure set by the Environmental Protection Agency. Thus, "safe" levels of pollutants for people are not considered "safe" for collections.
5 Light
|
|
Light energy, in the form of waves, is absorbed by molecules within an object. This absorption of energy activates a variety of chemical reactions that have the potential to damage paper-based collections. This is called photochemical deterioration.
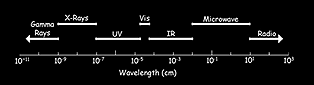
Visible light falls between 400 and 760 nanometers on the electromagnetic spectrum (violet to red). Wavelengths below 400 nanometers (ultraviolet, or UV, light) have a greater frequency than visible light, and bombard an object with more energy in a shorter time, accelerating photochemical deterioration. At the other end of the spectrum, infrared light (IR, above 760 nanometers) causes heating, thermal expansion, and spot desiccation. Fundamentally, although some types of light may be worse than others, all light is damaging, including visible light.
Sources of Light
Collections may be exposed to both natural and artificial light. Natural light is high in both UV and IR light, while artificial lighting varies.
There are multiple types of artificial light sources. Traditional tungsten incandescent bulbs are high in IR but do not emit much UV. However, tungsten halogen incandescent bulbs (also called quartz iodine) may have high levels of UV. Discharge bulbs include compact fluorescent tubes (CFTs, which are the type of lighting most frequently seen in collections storage), compact fluorescent lamps (CFLs, made in various shapes to fit into traditional incandescent fixtures) and metal halide bulbs. Metal halide bulbs emit high levels of UV, as do many CFTs and CFLs.
In fiber optic lighting, optical fibers carry the light from a single light source to various locations (this is often used for exhibit lighting). Fiber optic lighting may emit some IR light, but any UV in the original light source is removed as the light passes through the fibers.
Light Emitting Diodes (LEDs) are semiconductor devices (materials with electrical conductivity) that emit a specific spectrum or color temperature of light depending on the semiconductor material used. LED lights do not emit UV or IR radiation, or heat, although their conductor box does give off heat. LEDs can supply light for a lower energy cost and have a longer lifespan than other light sources.
6 Guidelines for Light Levels
|
|
Certainly the most protection is provided by reducing light exposure completely, through storage in boxes or other enclosures. Light levels of 200 to 600 lux are required for comfortable vision in study and inspection areas, but storage areas can and should be as low as 10 to 50 lux. Lights should be turned off whenever possible. Ideally sunlight should be excluded completely from storage and use areas, or shades, drapes, and/or blinds should be used to reduce exposure as much as possible.
Due to the diversity of materials and their responses to light exposure, there is no standard for light levels during exhibition and use. In general, exhibition lighting for very light sensitive materials (this includes most paper-based items) should be limited to 50 lux, and exhibition time should be limited. Further information on exhibition can be found in Session 3: Caring for Collections.